GelRed Nucleic Acid Gel Stain, 10,000x in Water
Catalog Number: 41003
Packaging Size: 0.5ml
Catalog Number: 41003-1
Packaging Size: 10ml
Storage and Handling:
GelRed is a very stable dye. Store 10,000x solution and dilute solutions of GelRed at room temperature, protected from light. Dye precipitation may occur at lower temperatures, resulting in lower signal, or the appearance of precipitate on the surface of the gel. If this occurs, heat the GelRed solution to 45-50o for two minutes and vortex. GelRed is stable for at least one year from the date it is received.
Product Description:
GelRed is a sensitive, stable and environmentally safe fluorescent nucleic acid dye designed to replace the highly toxic ethidium bromide (EtBr) for staining dsDNA, ssDNA or RNA in agarose gels or polyacrylamide gels. GelRed and EtBR have virtually the same spectra (Figure 1) so you can directly replace EtBr with GelRed without changing your existing imaging system.
In addition, GelRed is far more sensitive than Ethidium Bromide (Figure 2).
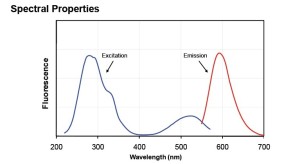 Figure 1: Excitation (left) and emission (right) spectra of GelRed bound to dsDNA in TBE.
Figure 1: Excitation (left) and emission (right) spectra of GelRed bound to dsDNA in TBE.
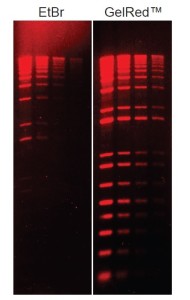
Figure 2: GelRed is more sensitive than EtBR. Comparison of GelRed and ethidium bromide (EtBr) in precast gel staining using 1% agarose gel in TBE buffer. Twofold serial dilutions of 1kb Plus DNA Ladder from Invitrogen were loaded onto each gel in 4 lanes in the amounts of 200ng, 100ng, 50ng and 25ng, respectively from left to right.
GelRed™ was subjected to a series of tests at Biotium and by three independent testing services to assess the dye’s safety for routine handling and disposal. Test results confirm that the dye is impenetrable to both latex gloves and cell membranes. The dye is noncytotoxic and nonmutagenic at concentrations well above the working concentrations used in gel staining. GelRed™ successfully passed environmental safety tests in compliance with CCR Title 22 Hazardous Waste Characterization, under which GelRed™ is not classified as hazardous waste. A complete safety report is available at www.biotium.com.
Although GelRed™ has undergone extensive safety testing, Biotium recommends following universal safety precautions when working in the laboratory.
Staining Protocols
Because high affinity nucleic acid binding dyes can affect DNA migration during electrophoresis, post-staining of gels is highly recommended. Post-staining with GelRed results in superior sensitivity and eliminates the possibility of dye interference with DNA migration. Agarose gels can be precast with GelRed™, however, GelRed™ may affect the migration or resolution of some DNA samples in precast gels. The precast protocol is not recommended for polyacrylamide gels.
GelRed can be used to stain dsDNA, ssDNA or RNA, however GelRed is twice as sensitive for dsDNA than ssDNA or RNA. Gel staining with GelRed™ is compatible with downstream applications such as sequencing and cloning. GelRed is efficiently removed from DNA by phenol/chloroform extraction and ethanol precipitation.
1. Post-Staining Protocol
1.1 Run gels according to your standard protocol.
1.2 Dilute GelRed 10,000X stock solution 3,300 fold to make a 3X staining solution in H2O. Generally 50 mL staining solution is an adequate volume for one minigel. Note: including 0.1 M NaCl in the staining solution enhances sensitivity, but may promote dye precipitation if the gel stain is reused.
1.3 Place the gel in a suitable container such as a polypropylene staining tray. Add a sufficient amount of the 3X staining solution to submerge the gel.
1.4 Agitate the gel gently at room temperature for ~30 minutes. Note: Optimal staining time may vary somewhat depending on the thickness of the gel and the percentage of agarose. For polyacrylamide gels containing 3.5-10% acrylamide, typical staining time is 30 minutes to 1 hour with gels of higher acrylamide content requiring longer staining time.
1.5 Destaining is not required, but the gel can be washed in water to reduce background if necessary.
1.6 View the stained gel with a standard transilluminator (302 or 312 nm) and image the gel using an ethidium bromide filter. SYBR or GelStar filters also may be used for gel imaging with equally good results.
1.7 Staining solution can be reused at least 2-3 times. Store staining solution at room temperature protected from light.
2. Precast Protocol for Agarose Gels
2.1 Prepare molten agarose gel solution using your standard protocol.
Note: the precast protocol is not recommended for polyacrylamide gels. Polyacrylamide gels can be stained using the post-stain protocol.
2.2 Dilute the GelRed 10,000X stock reagent into the molten agarose gel solution at 1:10,000 and mix thoroughly. GelRed can be added while the gel solution is still hot.
2.3 Cast the gel and allow it to solidify.
2.4 Load samples and run the gels using your standard protocol.
2.5 View the stained gel using a standard transilluminator (302 or 312 nm) and image the gel using an ethidium bromide filter. SYBR or GelStar filters also can be used for gel imaging with equally good results.
2.6 Unused agarose containing GelRed™ can be remelted to cast more gels, but it may be necessary to add more dye for optimal signal. We do not recommend storing agarose containing GelRed™ in molten form (i.e., at 50ºC) for more than a few days. Precast gels containing GelRed™ can be stored at 4ºC for future use.
Troubleshooting:
Problem:
Smeared DNA bands in precast gel.
Suggestion:
1. Reduce the amount of DNA loaded by one-half to one-third. GelRed is much more sensitive than EtBr. Blown out or smeared bands can be caused by overloading. This is frequently observed with DNA ladders. Biotium offers a 1kb ladder that has been optimized for use with GelRed (see related products below).
2. Perform post-staining instead of pre-casting.
3. Pour a lower percentage agarose gel for better resolution of large fragments.
4. Change the running buffer. TBE buffer has a higher buffering capacity than TAE.
5. Loading buffers containing SDS may contribute to bad smearing. If this occurs, use the post-staining protocol for applications requiring SDS-containing loading buffers.
Problem:
Discrepant DNA migration in pre-cast gel.
Suggestion:
GelRed is designed to be larger than other dyes to prevent it from entering cells, thus rendering the dye safer. The migration of DNA may be affected depending on the dye:DNA ratio.
1. Reduce the amount of DNA loaded by one-half to one-third.
2. Reduce the amount of dye used; ie use 0.5X in precast gels.
3. Post-stain gel in 3X GelRed to avoid any interference the dye may have on migration during electrophoresis.
Problem:
Weak fluorescence, decreased dye performance over time, or film of dye remains on gel after post-staining.
Suggestion:
The dye may have precipated out of solution.
1. Heat GelRed solution to 45-50°C for two minutes and vortex to redissolve.
2. Store dye at room temperature to avoid precipitation.
GelRedTM and its uses are covered by US patent numbers 796048, 7803943, and 8232050.
Materials from Biotium are sold for research use only, and are not intended for food, drug, household or cosmetic use.
© Copyright 2023 Gene Target Solutions Pty Ltd